I mentioned a few days ago about the recent change in the antihistamine Allegra, as you can now buy it without a prescription. But as one of my colleagues mentioned to me, the generic is now available, also without a prescription.
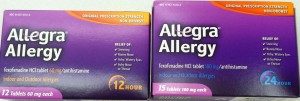
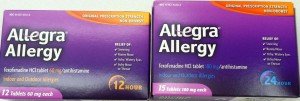
Drug Safety Tip: Be sure to look carefully at the box of Allegra or fexofenadine before you buy it…there are 2 different tablet strengths sold on the same shelf. The 180mg strength of Allegra or fexofenadine is labeled 24-Hour. The other strength (the 60 mg) is labeled 12-Hour.
Now, you might already be thinking, “Taking 60mg Allegra twice a day only gives you 120mg per day, instead of 180mg. Hmmm…why wouldn’t it be a 60mg tablet every 8 hours http://homepage.westmont.edu/make_account/images/pic/acrobat-xi-standard.html visit this link?”
Good question.
The answer is that the dose of 60mg every 12 hours and 180mg every 24 hours were the doses originally approved by the FDA for the prescription form of Allegra, and had already been approved for non-Rx status.
When the company applied for approval to sell Allegra to consumers, the FDA approved the same doses as the prescription forms had. AND by the way, Allegra was approved as safe for NON-prescription use years ago by an FDA committee. That particular advisory committee decided that Allegra, as well as Claritin, was safe enough to sell directly to consumers. But at that time Allegra and Claritin were VERY, VERY PROFITABLE prescription products for their companies Sanofil-Aventis and Schering-Plough, and those companies chose to keep their profitable products prescription only. Well, until their patent protection expired, anyway.
And now you can purchase Allegra, Claritin and their generic equivalents fexofenadine and loratidine OTC (over the counter), just in time for ragweed season.